Index |
 |
|
|
Materials - Levels 3 - 6 - Answers
[ Questions ] |
| | |
|
Fill in the missing word. When a solid dissolves in a __ solvent __ a solution is made.
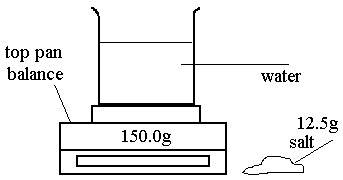
John adds 12.5g of salt to the beaker and stirs until the salt dissolves in the water.
|
|
What is the reading when all the salt has dissolved? _____ 162.5g _____
|
|
Describe how John could get all the salt back as a solid form using the salt solution he has made.
heat/warm/ evaporate solution until all water has gone or until dry
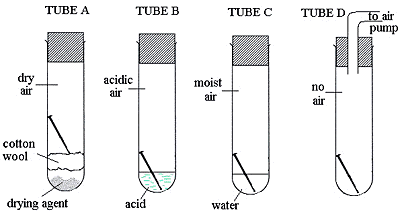 Four test tubes were set up as shown, with a shiny new, steel nail in each.
|
|
Write down the letters of the tubes in which the nail will not rust.
__ A __ and __ D ___
|
|
In which tube will the nail rust the fastest? __________ B ____________
|
|
Bikes have many steel parts. Suggest two ways to prevent these parts from going rusty. paint / cover with grease or oil / chrome plate
Study the flow chart below. Each arrow represents a reaction.
|
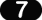
|
To leave just zinc, an element has to be removed from zinc oxide. Name the element removed.
________ oxygen ___________
|
|
Zinc is treated with an acid to make zinc sulphate. Which acid is used?
_____________ sulphuric acid ____________
|
|
When zinc dissolves in an acid, bubbles of hydrogen gas are given off. How would you test for hydrogen and how do you know if it is present?
Collect gas in a test tube. Try to light gas with a Bunsen or burning splint. If hydrogen is present it burns with blue flame and a sharp pop.
|





|



|
| |
 |
| |
Copyright©1998 - British Telecommunications plc |
| |
Last modified on: Tuesday, June 9, 1998.
|