Index |
 |
|
|
Materials - Levels 3 - 6
[ Answers ] |
| | |
|
Fill in the missing word. When a solid dissolves in a ___________ a solution is made.
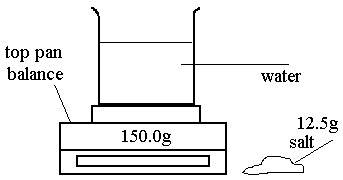
John adds 12.5g of salt to the beaker and stirs until the salt dissolves in the water.
|
|
What is the reading when all the salt has dissolved?
|
|
Describe how John could get all the salt back as a solid form using the salt solution he has made.
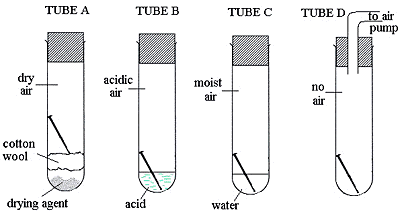 Four test tubes were set up as shown, with a shiny new, steel nail in each.
|
|
Write down the letters of the tubes in which the nail will not rust.
_____ and _____
|
|
In which tube will the nail rust the fastest? ___________________________
|
|
Bikes have many steel parts. Suggest two ways to prevent these parts from going rusty. __________________________
___________________________
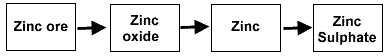
Study the flow chart below. Each arrow represents a reaction.
|
|
To leave just zinc, an element has to be removed from zinc oxide. Name the element removed.
______________________
|
|
Zinc is treated with an acid to make zinc sulphate. Which acid is used?
_____________________________
|
|
When zinc dissolves in an acid, bubbles of hydrogen gas are given off. How would you test for hydrogen and how do you know if it is present?
|





|



|
| |
 |
| |
Copyright©1998 - British Telecommunications plc |
| |
Last modified on: Tuesday, June 9, 1998.
|