
Materials - Levels 5 - 7 - Answers
[ Questions ]
| drink/ food | pH |
| Pork | 7 |
| Peaches | 4 |
| orange juice | 2 |
| cola | 3 |
When cans were first used over a hundred years ago, it was found that meat such as pork kept for months even in the un-plated steel cans of the time. However, steel cans of peaches bulged, or even burst because of a gas produced inside.
acid in peaches reacts with iron /steel
hydrogen
The list shows part of the reactivity series of metal. (The most reactive is at the top).
Sodium
Magnesium
Aluminium
Zinc
Iron (steel is mostly iron)
Tin
Copper
Gold
tin is less reactive than iron and steel
crack in tin allows chemicals in food to contact the iron and steel and a reaction may occur
Aluminium is more reactive than iron, therefore you would expect it to be less suitable for cans than iron. However, aluminium reacts with oxygen in the air to produce aluminium oxide. This creates an inert layer that stops the aluminium affecting the drink.
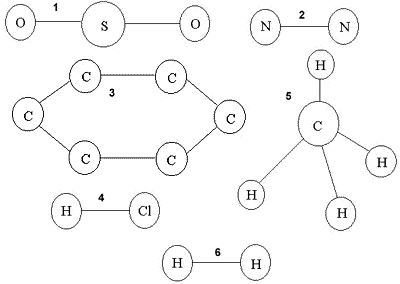
The diagrams above show how atoms are arranged inside six substances. Each circle represents an atom and the letter inside is the chemical symbol.
1, 4, 5
They contain more than one element
5 is CH4 , 6 is H2
_____________________________
Cl ____ chlorine ___
Mg ___ magnesium ___
Na ____ sodium ____
S _____ sulphur ____
sulphur dioxide
