

Luminescence is the emission of light by a substance. It occurs when an electron returns to the electronic ground state from an excited state and loses it's excess energy as a photon.
Luminescence spectroscopy is a collective name given to three related spectroscopic techniques. They are;
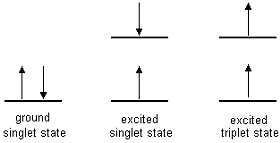
| Singlet state: All electrons
in the molecule are spin-paired Triplet state: One set of electron spins is unpaired |
|
Fluorescence
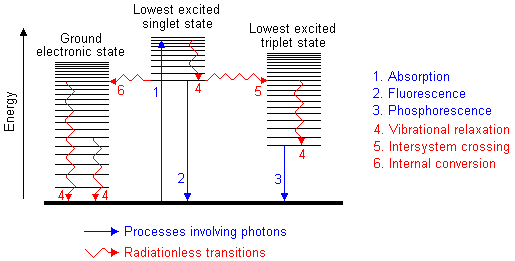
Absorption of UV radiation by a molecule excites it from a vibrational level
in the electronic ground state to one of the many vibrational levels in the
electronic excited state. This excited state is usually the first excited singlet
state. A molecule in a high vibrational level of the excited state will quickly
fall to the lowest vibrational level of this state by losing energy to other
molecules through collision. The molecule will also partition the excess energy
to other possible modes of vibration and rotation. Fluorescence occurs when
the molecule returns to the electronic ground state, from the excited
singlet state, by emission of a photon. If a molecule which absorbs UV radiation
does not fluoresce it means that it must have lost its energy some other way.
These processes are called radiationless transfer of energy. Have a look
at this diagram:
Intra-molecular redistribution of energy between possible electronic and
vibrational states
The molecule returns to the electronic ground state.The excess energy is converted
to vibrational energy (internal conversion), and so the molecule is placed
in an extremely high vibrational level of the electronic ground state. This
excess vibrational energy is lost by collision with other molecules (vibrational
relaxation). The conversion of electronic energy to vibrational energy is
helped if the molecule is "loose and floppy", because it can reorient itself
in ways which aid the internal transfer of energy.
A combination of intra- and inter-molecular energy redistribution
The spin of an excited electron can be reversed, leaving the molecule in an
excited triplet state; this is called intersystem crossing. The
triplet state is of a lower electronic energy than the excited singlet state.
The probability of this happening is increased if the vibrational levels of
these two states overlap. For example, the lowest singlet vibrational level
can overlap one of the higher vibrational levels of the triplet state. A molecule
in a high vibrational level of the excited triplet state can lose energy in
collision with solvent molecules, leaving it at the lowest vibrational level
of the triplet state. It can then undergo a second intersystem crossing to a
high vibrational level of the electronic ground state. Finally, the molecule
returns to the lowest vibrational level of the electronic ground state by vibrational
relaxation.
Phosphorescence
A molecule in the excited triplet state may not always use intersystem crossing
to return to the ground state. It could lose energy by emission of a photon.
A triplet/singlet transition is much less probable than a singlet/singlet transition.
The lifetime of the excited triplet state can be up to 10 seconds, in comparison
with 10-5 s to 10-8 s average
lifetime of an excited singlet state. Emission from triplet/singlet transitions
can continue after initial irradiation. Internal conversion and other radiationless
transfers of energy compete so successfully with phosphorescence that it is
usually seen only at low temperatures or in highly viscous media.
Chemiluminescence occurs when a chemical reaction produces an electronically excited species which emits a photon in order to reach the ground state. These sort of reactions can be encountered in biological systems; the effect is then known as bioluminescence. The number of chemical reactions which produce chemiluminescence is small. However, some of the compounds which do react to produce this phenomenon are environmentally significant.
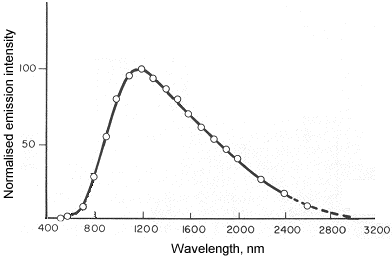
A good example of chemiluminescence is the determination of nitric oxide:
NO + O3 ® NO2* + O2
NO2* ® NO2 + hv (l = 600 - 2800 nm)
The following graph shows the spectral distribution of radiation emitted by the above reaction:
You should understand the processes of fluorescence and phosphorescence, and which properties of a molecule make it likely to be photoluminescent.You should also be aware of the various ways in which an excited molecule can return to the ground state without emission of radiation.
Why is chemiluminescence spectroscopy a highly selective, sensitive and simple
technique?
Hints: How common are chemiluminescent reactions? Is the emitted radiation measured
against a noisy background?
|
|
|

 Biosciences Homepage
Biosciences Homepage
 |