

wavenumber = 1 / wavelength in centimetersIt is useful to divide the infra red region into three sections; near, mid and far infra red;
| Region | Wavelength range (mm) | Wavenumber range (cm-1) |
| Near | 0.78 - 2.5 | 12800 - 4000 |
| Middle | 2.5 - 50 | 4000 - 200 |
| Far | 50 -1000 | 200 - 10 |
For a molecule to absorb IR, the vibrations or rotations within a molecule must cause a net change in the dipole moment of the molecule. The alternating electrical field of the radiation (remember that electromagnetic radation consists of an oscillating electrical field and an oscillating magnetic field, perpendicular to each other) interacts with fluctuations in the dipole moment of the molecule. If the frequency of the radiation matches the vibrational frequency of the molecule then radiation will be absorbed, causing a change in the amplitude of molecular vibration.
Molecular rotations
Rotational transitions are of little use to the spectroscopist. Rotational levels are quantized, and absorption of IR by gases yields line spectra. However, in liquids or solids, these lines broaden into a continuum due to molecular collisions and other interactions.
Molecular vibrations
The positions of atoms in a molecules are not fixed; they are subject to a number of different vibrations. Vibrations fall into the two main catagories of stretching and bending.
Stretching: Change in inter-atomic distance along bond axis
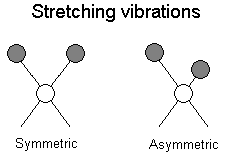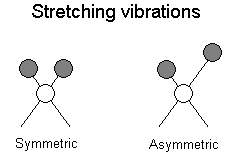
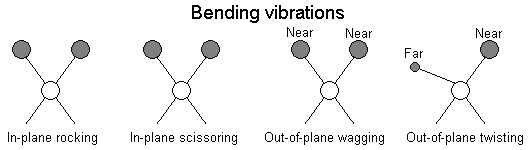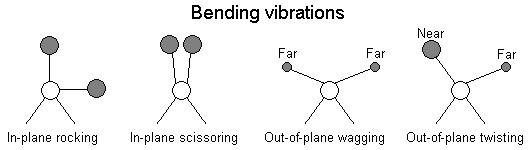
In addition to the vibrations mentioned above, interaction between vibrations can occur (coupling) if the vibrating bonds are joined to a single, central atom. Vibrational coupling is influenced by a number of factors;
You should now understand how certain molecules absorb infra red radiation, and the effects that this absorption has. You should be familiar with the ways in which molecules can vibrate, and factors which influence how these vibrations interact with each other.
|
|
|

 Biosciences Homepage
Biosciences Homepage
 |